Overview
As Mercer Rang has correctly pointed out, "Children are not young adults." Children differ significantly from adults with respect to skeletal anatomy and physiology. Differences in bone growth and modeling, as well as remodeling, affect the way in which conditions involving the skeleton should be viewed and managed.
The skeletal anatomy of children and toddlers (see the images below) differs from the skeletal anatomy of adults. The following are some of the key differences.
Bone in children and toddlers is more porous than adult bone, with wider haversian canals.
A child's bones are more elastic than an adult's are. Two terms are important here: plasticity and elasticity. Bones in children permit a greater degree of deformation before they break. At times, the bone may deform but not fracture, a condition often described as a plastic deformation. At other times, the bone may simply buckle to create what is described as a torus fracture. These patterns are not seen in adults, in whom the bones' resistance and elasticity to angular deformation is significantly less.
The periosteal sleeve (see the image below) is much thicker in children than in adults and acts as a restraint to displacement. Angular deformation of a child's bone may cause fracture of the cortices without displacement ("greenstick" fracture). The thick periosteal sleeve is important for pediatric skeletal remodeling. Subperiosteal resection of a child's bone shows the regeneration potential associated with the periosteum: the tubular bone eventually reforms inside the periosteal sleeve. Thus, the child's bone has an innate potential to heal itself.
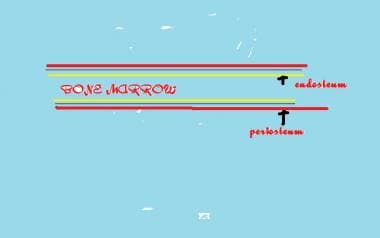 Basic structure of long bone. Outer covering is periosteum, inner lining is endosteum, and contents are bone marrow.
Basic structure of long bone. Outer covering is periosteum, inner lining is endosteum, and contents are bone marrow.
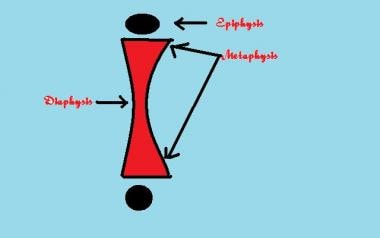
The epiphysis is an important part of the growing skeleton. It is a secondary ossification center. Mercer Rang emphasizes the importance of using accurate terminology in relation to these parts of the growing skeleton. The physis is to the growth plate, which is a disklike structure at the end of the metaphysis; the epiphysis is a cartilaginous structure that sits atop the physis.
The growing skeleton responds to injury and infection differently from the adult skeleton. Conditions that affect the physis and the growth disturbances that may result can create challenging issues in management.
Microscopic Anatomy
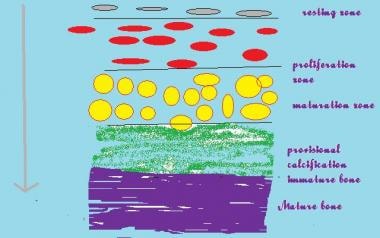
The epiphysis is the growing end of the long bone and is responsible for an increase in the length of the long bones (see the image below).
Bones are formed by 1 of the following 2 processes:
- Endochondral ossification - This involves the formation of bone from a cartilaginous anlage; long bones are primarily formed by endochondral ossification
- Membranous ossification - The cartilaginous phase is usually absent; ossification of flat bones and skull bones is usually via this process
Histologically, the physis consists of a number of layers that reflect the process of bone formation. The basal layer consists of resting cartilage cells. These multiply under the influence of growth factors in the zone of multiplication, and rounded cells are then seen. These rounded cells arrange themselves in rows and mature in a loose cartilaginous matrix.
Blood vessels from the metaphysis (see the image below) invade this zone to lay down minerals into the matrix, and loose woven bone is then laid down in the zone of provisional calcification. Mature bone is finally laid down in the metaphysis. A continuation of this process leads to an increase in the length of the long bone.
The epiphysis and the metaphysis are connected by mammillary processes internally and by the tough fibrous periosteum externally, both of which resist displacement forces. This attachment is not rigid and allows microscopic translation forces, and this flexibility protects the structure from injury.
Rang states that the epiphysis is periarticular and that forces typically causing dislocation in the adult are likely to cause epiphyseal or physeal injury in the child.
Remodeling
Remodeling of a fracture or deformity is a process that is carried out more efficiently in the child than in the adult. A deformity corrects itself by asymmetrical appositional formation of new bone. Remodeling is influenced by a number of factors, including the following:
- Age - The younger the age, the better the remodeling potential
- Proximity to the physis - Fractures closer to the physis remodel better than those away from the physis
- Relation to the axis of joint motion - Deformities in the axis of joint motion remodel better than deformities outside the axis of joint motion
- Rotational versus nonrotational deformity - Rotational deformities do not remodel and correct themselves
An injury to a long bone can stimulate excessive growth and effectively create a temporary limb length discrepancy. The most common example is the stimulation of growth at the proximal femur after a fracture in the shaft of the femur. This phenomenon allows the surgeon to accept some shortening in the treatment of these fractures, given that they would be expected to correct with time.
In contrast, a physeal injury can cause severe growth arrest and lead to limb length discrepancies and deformities that can require years of treatment to correct. The most devastating of these injuries is seen in the aftermath of pediatric infections. Septic arthritis of the hip leads to severe limb length discrepancies and loss of function and stability.
Natural Variants
Bone in children undergoes serial changes and adaptations to achieve its adult form over a period of years as the child reaches maturity.
The secondary ossification centers appear at various ages; these can be a guide to bone age and true skeletal age and thus are often helpful in resolving forensic and medicolegal issues. The fusion of these secondary ossification centers also follows a set pattern, which is also useful in skeletal age determination.
For example, the ossification center of the greater trochanter appears at the age of 3 in girls and 6 in boys, whereas that of the lesser trochanter appears at the age of 6 in both girls and boys. The secondary ossification centers for the pubis appears at 9-11 years in girls and at 13-16 years in boys. [1]
At birth, the sutures in the skull are unfused at the anterior and posterior fontanelles. However, whereas the posterior fontanelle fuses soon after birth, the anterior fontanelle may not close until much later (eg, 12-18 months). Delayed closure of this fontanelle is seen in malnutrition and in rickets. Premature and rigid fusion of skull bones is seen in craniosynostosis, which requires complex multidisciplinary procedures to correct.
The alignment of the lower limbs also changes as the child progresses from crawling to the unsteady bipedal gait of the toddler and, finally, to the established bipedal gait of the child.
The toddler demonstrates a varus alignment at the knee and a waddle in the gait; the foot arches are yet not developed. By about 2 years of age, knee alignment of the knee becomes neutral. Over the next few years, knee alignment progresses to a physiologic genu valgum, which spontaneously corrects itself to a normal tibiofemoral alignment by about 7 years of age. These variations are important to understand because parents often consult an orthopedist for these issues, which require nothing more than careful clinical examination and reassurance.
The varus and valgus alignments are believed to be dictated by the relative growth rates of the articular cartilage and the adjacent physeal zone. The physis grows almost 5 times faster than the articular cartilage does. This difference may control varus and valgus alignment: varus develops if the medial sides grow more slowly than the lateral sides, and valgus develops if they grow more quickly. [2]
Gender and racial differences are well known. A number of studies have documented the evolution of the tibiofemoral angle in children by using the tibiofemoral measurement and measurements of intercondylar and intermalleolar distances. A study of European children showed that boys had a greater tendency toward varus than girls did at the end of development, with lower intermalleolar and intercondylar distances. [3]
A study from Turkey showed that Turkish children had a higher valgus angle than children assessed in other reports in the literature. [4]
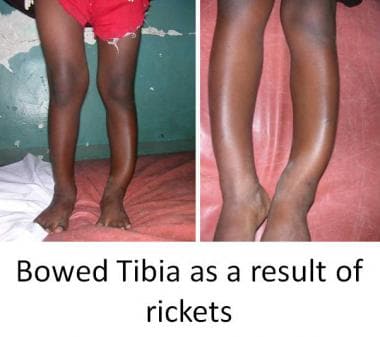
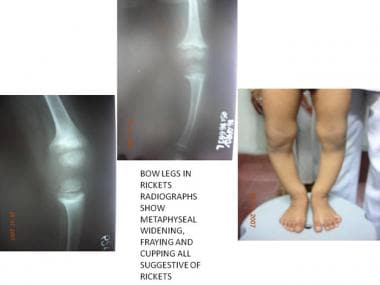
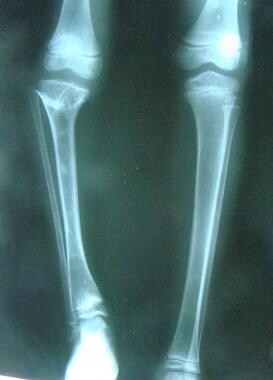
Pathologic variations can occur in the growth process and can alter skeletal anatomy and physiology in children. Faulty mineralization of the osteoid manifests itself as rickets. This may occur as a result of a nutritional deficiency of vitamin D or a more complex deficiency related to faulty renal tubular function. Rickets weakens the bone, leading to deformities that may necessitate correction, such as genu varum, genu valgum, and tibial bowing (see the images below).
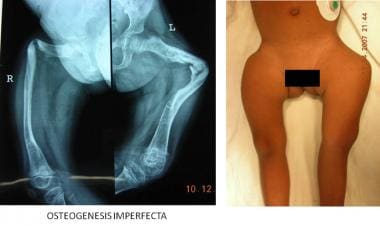
Matrix deficiencies can give rise to a myriad of conditions, the best known of which is osteogenesis imperfecta, which can lead to multiple pathologic fractures and deformities in childhood (see the image below). Deficiencies of growth hormone and thyroid dysfunction also lead to stunting of growth and dwarfism.
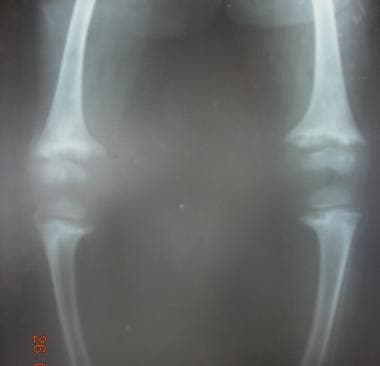
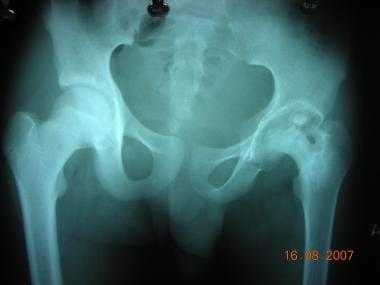
Infections and trauma can cause complete or partial physeal arrest, causing deformities and limb length discrepancies (see the images below). Epiphyseal dysplasias also cause stunted growth and deformities.

No comments:
Post a Comment