So let Look At Fluid, Real Women Drink Fluid When "Pregnant" And" Real Mean And Real Women Drink Fluids When We Get Sick Or Are They Talking About Pressure in Fluids?, MAY Be They Mean Fluid Mechanics That Got To Be It , You Know Mechanical Engineering? , Come Think About It, It's Going To Be, The Fluid Mosaic Model , That it, There It Is! , No Wait A Minute, I Got It,There Talking About, Fluid In Lungs, That Got To Be It! We Found It! Party Time! , Nope Fluid In Lungs Was Not It, Well Were S.J.W Come With Gender-Fluid, Crap, There Just No Science To Prove It!

1. Real Women, The Institute of Medicine recommends that pregnant women drink about 10 8-ounce cups of water or other beverages each day.
Women's needs differ, so don't worry if you find yourself needing a bit more or less. You can tell you're getting enough if your urine looks pale yellow or colorless. You're likely to need more fluid than usual in a warm climate, at high altitude, or if you're exercising.
Plain water is an ideal beverage, but milk, juice, coffee, and tea all contain plenty of water and count toward your fluid intake. Keep in mind, though, that juice and sweetened drinks also provide a lot of extra calories, so you don't want to rely on them too much.
It's best to limit caffeine, too, including caffeinated coffee, teas, and sodas. The American College of Obstetricians and Gynecologists (ACOG) advises pregnant women to get no more than 200 milligrams of caffeine per day.
Don't hesitate to drink water and other fluids because you're afraid of retaining water. Oddly enough, fluid retention can result from not drinking enough, because your body will hang on to more fluid if it senses it's becoming dehydrated.
So if your feet and ankles are swollen, drinking more water can actually help. (If swelling is excessive or comes on suddenly, contact your caregiver, as this can be a sign of preeclampsia.)
Fluids also help prevent common pregnancy problems such as constipation, hemorrhoids, and bladder infections. (Drinking water dilutes your urine, which reduces your risk of infection.)
It's especially important to stay hydrated in the last trimester, when dehydration can cause contractions that can trigger preterm labor.
Ten cups might seem like a lot, especially if you're battling nausea during pregnancy. Try sipping water throughout the day rather than drinking a lot at once. If you don't like the taste of water, try adding a lemon or lime wedge or a little juice for additional flavor.
2. Real Mean And Real Women fluids to drink when sick.
Best (and Worst) Drinks When You Have a Cold
When you're sick, you hear it over and over: "Get plenty of fluids.” And it’s true. Your body needs extra hydration when you’re trying to get well.
But what, exactly, should you put in your cup? Some drinks are great for easing symptoms, and others may make them worse. Keep these tips in mind when you’re picking what to sip.
Drinks That Help
- Decaf tea. The heat can soothe sore throats, stuffy noses, chest congestion, and upset stomachs. Plus, a warm cup of tea is comforting when you’re feeling rough. If you choose one with herbs like ginger, your immune system may get a small boost, too. For an extra benefit, try adding a small dollop of honey to your cup: it can calm a cough and help you sleep.
- Water with lemon. Hot or cold, it keeps you hydrated and loosens up stuffiness and congestion. Lemon is also high in vitamin C, which may make a cold slightly shorter if you get it regularly.
- Soup. Clear soups and broths give you calories when you may not have much of an appetite. Some research shows they may help relieve inflammation in the body, which can relieve some of your symptoms. The warmth also helps break up mucus.
- Ice chips or ice pops. True, these aren’t technically a beverage, but they have the same purpose: keeping you hydrated.
What You Don’t Need
These drinks won’t help you get over your cold or flu, and some could do more harm than good.
- Sports drinks. They can help if you’re very dehydrated, but they don't really do much to make you feel better. Plus, they have a lot of sugar. Other drinks will help you hydrate without the extra sweet stuff.
- Fruit juices. Juice may seem like a good idea, especially for kids, but like sports drinks, most have loads of added sugar. Citrus-based ones like orange juice can also aggravate sore throats. If it's all your child wants to drink, try adding a splash or two to a cup of water instead of a glassful of juice.
- Coffee. If you’re going to sip a hot drink, might as well get your daily dose of caffeine in it, right? Wrong. Caffeine can make you dehydrated, which isn’t good when you’re trying to get well. The same goes for soda and caffeinated teas.
- Ginger ale. While ginger in its natural form may have some cold and flu benefits, this carbonated, sugary version won't offer much relief. Soft drinks of any kind have very little of the nutrients and electrolytes you need to fight off sickness. Get your ginger in a mug of hot tea instead.
- Alcohol. It dehydrates you and can make some symptoms worse, like nausea, headaches, and body aches. Booze can also make your body less able to handle infections. So save that hot toddy for when you’re feeling better.
- http://symptoms.webmd.com/cold-flu-map/how-to-stay-hydrated-when-youre-sick
3.How do you find the pressure in a fluid?
What does pressure mean?
If you tried to hammer a bowling pin into the wall, nothing would probably happen except for people deciding to no longer lend you their bowling pins. However, if you hammer with the same force on a nail, the nail would be a lot more likely to penetrate the wall. This shows that sometimes just knowing the magnitude of the force isn't enough, you also have to know how that force is distributed on the surface of impact. For the nail, all the force between the wall and the nail was concentrated into the very small area on the sharp tip of the nail. However, for the bowling pin the area touching the wall was much larger, and therefore the force was much less concentrated.
To make this concept precise, we use the idea of pressure. Pressure is defined to be the amount of force exerted per area.
So to create a large amount of pressure, you can either exert a large force or exert a force over a small area (or do both). In other words, you might be safe lying on a bed of nails if the total surface area of all the nail tips together is large enough.
This definition also means that the units of pressure are newtons per square meter
which are also called pascals or abbreviated as .
How do you find the pressure in a fluid?
A solid surface can exert pressure, but fluids (i.e. liquids or gases) can also exert pressure. This might seem strange if you think about it because it's hard to imagine hammering in a nail with liquid. To make sense of this, imagine being submerged to some depth in water. The water above you would be pushing down on you because of the force of gravity and would therefore be exerting pressure on you. If you go deeper, there will be more water above you, so the weight and pressure from the water would increase too.
Not only can the weight of liquids exert pressure, the weight of gases can as well. For instance, the weight of the air in our atmosphere is substantial and we're almost always at the bottom of it. The pressure exerted on your body by the weight of the atmosphere is surprisingly large. The reason you don't notice it is because the atmospheric pressure is always there. We only notice a change in pressure above or below normal atmospheric pressure (like when we fly in an airplane or go underwater in a pool). We aren't harmed by the large atmospheric pressure because our body is able to exert a force outward to balance the air pressure inward. But this means that if you were to be thrown into the vacuum of outer space by space pirates, your body pressure would continue pushing out with a large force, yet no air would be pushing in.
Okay, so the weight of a fluid can exert pressure on objects submerged in them, but how can we determine exactly how much pressure a fluid will exert? Consider a can of beans that got dropped in a pool as seen in the following diagram.
The weight of the column of water above the can of beans is creating pressure at the top of the can. To figure out an expression for the pressure we'll start with the definition of pressure.
For the force we should plug in the weight of the column of water above the can of beans. The weight is always found with , so the weight of the column of water can be written as where is the mass of the water column above the beans. We'll plug this into the equation for pressure above and get,
At this point it might not be obvious what to do, but we can simplify this expression by writing in terms of the density and volume of the water. Since density equals mass per volume , we can solve this for the mass of the water column and write where is the density of the water and is the volume of the water column above the can (not the entire volume of the pool). Plugging in for the mass of the water column into the previous equation we get,
At first glance this appears to have only made the formula more complex, but something magical is about to happen. We have volume in the numerator, and area in the denominator so we're going to try and cancel something here to simplify things. We know that the volume of a cylinder is where is the area of the base of the cylinder and is the height of the cylinder. We can plug in for the volume of water into the previous equation and cancel the areas to get,
Not only did we cancel the areas, we created a formula that only depends on the density of the water , the depth below the water , and the magnitude of the acceleration due to gravity . This is really nice since nowhere does it depend on the area, volume, or mass of the can of beans. In fact, this formula doesn't depend on anything about the can of beans other than the depth it is below the surface of the fluid. So this formula would work equally well for any object in any liquid. Or, you could use it to find the pressure at a specific depth in a liquid without speaking of any object being submerged at all. You'll often see this formula with the and the swapping places like this,
Just to be clear here, is always talking about the density of the fluid causing the pressure, not the density of the object submerged in the fluid. The is talking about the depth in the fluid, so even though it will be "below" the surface of fluid we plug in a positive number. And the is the magnitude of the acceleration due to gravity which is .
Now you might think, "OK, so the weight of the water and pressure on the top of the can of beans will push the can downward right?" That's true, but it's only a half truth. It turns out that not only does the force from water pressure push down on the top of the can, the water pressure actually causes a force that pushes inward on the can from all directions. The overall effect of the water pressure is not to force the can downward. The water pressure actually tries to crush the can from all directions as seen in the diagram below.
If it helps, you can think about it this way. When the can of beans fell into the water, it quite rudely displaced a large amount of water molecules from the region where the can is now. This caused the entire water level to rise. But water is pulled down by gravity which makes it want to try and find the lowest level possible. So the water tries to force itself back into the region of volume that it was displaced from in an effort to try and lower the overall height of the body of water. So, whether a can of beans (or any other object) is in the water or not, the water molecules are always being squashed into each other from the force of gravity as they try to lower the water level to the lowest point possible. The pressure in the formula is a scalar that tells you the amount of this squashing force per unit area in a fluid.
At this point, if you've been paying close attention you might wonder "Hey, there's air above the water right? Shouldn't the weight of the column of air above the column of water also contribute to the total pressure at the top of the can of beans?" And you would be correct. The air above the column of water is also pushing down and its weight is surprisingly large.
If you wanted a formula for the total pressure (also called absolute pressure) at the top of the can of beans you would have to add the pressure from the Earth's atmosphere to the pressure from the liquid .
We typically don't try to derive a fancy term like for the atmospheric pressure since our depth in the Earth's atmosphere is pretty much constant for any measurements made near land.
This means that the atmospheric pressure at the surface of the Earth stays relatively constant. The value of the atmospheric pressure at the surface of the Earth is stuck right around . There are small fluctuations around this number caused by variations in weather patterns, humidity, altitude, etc., but for the most part when doing physics calculations we just assume that this number is a constant and stays fixed. This means, as long as the fluid you're finding the pressure for is near the surface of the Earth and exposed to the atmosphere (not in some sort of vacuum chamber) you can find the total pressure (also called absolute pressure) with this formula.
What's the difference between absolute pressure and gauge pressure?
When measuring pressure, people often don't want to know the total pressure (which includes atmospheric pressure). People typically want to know the difference in some pressure from atmospheric pressure. The reason is that atmospheric pressure doesn't change much and it's almost always present. So including it in your measurements can feel a bit pointless at times. In other words, knowing that the air inside of your flat tire is at an absolute pressure of
isn't really all that useful (since being at atmospheric pressure means your tire's flat). The extra pressure in the tire above atmospheric pressure is what will allow the tire to inflate and perform properly.
Because of this, most gauges and monitoring equipment use what is defined to be the gauge pressure . Gauge pressure is the pressure measured relative to atmospheric pressure. Gauge pressure is positive for pressures above atmospheric pressure, zero at atmospheric pressure, and negative for pressures below atmospheric pressure.
The total pressure is commonly referred to as the absolute pressure . Absolute pressure measures the pressure relative to a complete vacuum. So absolute pressure is positive for all pressures above a complete vacuum, zero for a complete vacuum, and never negative.
This can all be summed up in the relationship between the absolute pressure , gauge pressure , and atmospheric pressure which looks like this,
For the case of finding the pressure at a depth in a non-moving liquid exposed to the air near the surface of the Earth, the gauge pressure and absolute pressure can found with,
Because the only difference between absolute pressure and gauge pressure is the addition of the constant value of atmospheric pressure, the percent difference between absolute and gauge pressures become less and less important as the pressures increase to very large values. (see the diagram below)
What's confusing about pressure?
People often want to plug in the density of the object submerged into the formula for gauge pressure within a fluid , but the density in this formula is specifically referring to the density of the fluid causing the pressure.
People often mix up absolute pressure and gauge pressure. Remember that absolute pressure is the gauge pressure plus atmospheric pressure.
Also, there are unfortunately at least 5 different commonly used units for measuring pressure (pascals, atmospheres, millimeters of mercury, etc). In physics the conventional SI unit is the pascal Pa, but pressure is also commonly measured in "atmospheres" which is abbreviated as . The conversion between pascals and atmospheres is, not surprisingly, since one atmosphere is defined to be the pressure of the Earth's atmosphere.
What do solved examples involving pressure look like?
Example 1: Finding the pressure from the feet of a chair
A fuchsia colored four legged chair sits at rest on the floor. Each leg of the chair has a circular foot with a radius of . The well engineered design of the chair is such that the weight of the chair is equally distributed on the four feet.
Find the pressure in pascals between the feet of the chair and the floor.
Example 2: Force on a submarine porthole
A curious seahorse is looking into the circular window of a submarine that is sitting at a depth of underneath the Mediterranean sea. The density of the seawater is . The window is circular with a radius of . The seahorse is impressed that the window does not break from the pressure caused by the weight of the seawater.
What is the magnitude of the force exerted on the surface of the circular submarine window from the weight of the water?
Note: We used the gauge pressure in this problem since the question asked for the force caused from "the weight of the water", whereas the absolute pressure would yield a force caused by the weight of the water and the weight of the air above the water.
4.Fluid Mechanics: What is Fluid Mechanics? Mechanical Engineering
- First, What is a fluid?
- Three common states of matter are solid, liquid, and gas.
- A fluid is either a liquid or a gas.
- If surface effects are not present, flow behaves similarly in all common fluids, whether gases or liquids.
- Example - The Penn State Sea Lion
Students in the Penn State Mechanical Engineering Department have designed and built a human powered submarine, named the "Sea Lion" as part of a national contest. In the preliminary stages of the design, back in the early 1990's, some wind tunnel testing was done on various hull shapes and fin shapes. Since the submarine moves below any surface effects, it was perfectly valid to run these tests in a wind tunnel (using air as the working fluid) rather than in water (the actual fluid in which the submarine moves). As will be discussed in a later learning module, drag and lift measurements must, of course, be scaled properly according to the rules of dimensional analysis. - Example - PSU Harrier experiments
Several years ago, Professor Cimbala had a research grant from NASA to study the interaction of the jet exhaust from a harrier aircraft with the ground, while the aircraft is in hover with a wind blowing. Model tests were conducted in both a wind tunnel and a water tunnel. It was perfectly valid to run these tests in either air or water, since there were no free surface effects to worry about. As will be discussed in a later learning module, the results in either case must be scaled properly according to the rules of dimensional analysis. - Formal definition of a fluid - A fluid is a substance which deforms continuously under the application of a shear stress.
- Definition of stress - A stress is defined as a force per unit area, acting on an infinitesimal surface element.
- Stresses have both magnitude (force per unit area) and direction, and the direction is relative to the surface on which the stress acts.
- There are normal stresses and tangential stresses.
- Pressure is an example of a normal stress, and acts inward, toward the surface, and perpendicular to the surface.
- A shear stress is an example of a tangential stress, i.e. it acts along the surface, parallel to the surface. Friction due to fluid viscosity is the primary source of shear stresses in a fluid.
- One can construct a free body diagram of a little fluid particle to visualize both the normal and shear stresses acting on the body:
Free Body Diagram, Fluid Particle at Rest:
Consider a tiny fluid element (a very small chunk of the fluid) in a case where the fluid is at rest (or moving at constant speed in a straight line). A fluid at rest can have only normal stresses, since a fluid at rest cannot resist a shear stress. In this case, the sum of all the forces must balance the weight of the fluid element. This condition is known as hydrostatics. Here, pressure is the only normal stress which exists. Pressure always acts positively inward. Obviously, the pressure at the bottom of the fluid element must be slightly larger than that at the top, in order for the total pressure force to balance the weight of the element. Meanwhile, the pressure at the right face must be equal to that on the left face, so that the sum of forces in the horizontal direction is zero.
[Note: This diagram is two-dimensional, but an actual fluid element is three-dimensional. Hence, the pressure on the front face must also balance that on the back face.]
Free Body Diagram, Fluid Particle in Motion:
Consider a tiny fluid element (a very small chunk of the fluid) that is moving around in some flow field. Since the fluid is in motion, it can have both normal and shear stresses, as shown by the free body diagram. The vector sum of all forces acting on the fluid element must equal the mass of the element times its acceleration (Newton's second law).
Likewise, the net moment about the center of the body can be obtained by summing the forces due to each shear stress times its moment arm. As the size of the fluid element shrinks to "zero," i.e. negligibly small, the shear stress acting on one face of the element must be the same magnitude as those acting on the other faces. Otherwise, there would be a net moment, causing the fluid element to spin rapidly!
[Note: To obtain force, one must multiply each stress by the surface area on which it acts, since stress is defined as force per unit area.]
- Definition of stress - A stress is defined as a force per unit area, acting on an infinitesimal surface element.
- Definition of shear stress - Shear stress is defined as a force per unit area, acting parallel to an infinitesimal surface element.
- Shear stress is primarily caused by friction between fluid particles, due to fluid viscosity.
- Fluids at rest cannot resist a shear stress; in other words, when a shear stress is applied to a fluid at rest, the fluid will not remain at rest, but will move because of the shear stress.
- For a good illustration of this, consider the comparison of a fluid and a solid under application of a shear stress: A fluid can easily be distinguished from a solid by application of a shear stress, since, by definition, a fluid at rest cannot resist a shear stress.

If a shear stress is applied to the surface of a solid, the solid will deform a little, and then remain at rest (in its new distorted shape). One can say that the solid (at rest) is able to resist the shear stress. 
Now consider a fluid (in a container). When a shear stress is applied to the surface of the fluid, the fluid will continuously deform, i.e. it will set up some kind of flow pattern inside the container. In other words, one can say that the fluid (at rest) is unable to resist the shear stress. That is to say, it cannot remain at rest under application of a shear stress. - Another way of saying this is: A fluid at rest cannot resist a shear stress.
- Note, however, that a fluid at rest can resist a normal stress.
- The dictionary says mechanics is " ... the application of the laws of force and motion ... There are two branches, statics and dynamics. ..."
- Fluid Statics or hydrostatics is the study of fluids at rest. The main equation required for this is Newton's second law for nonaccelerating bodies, i.e.
 .
.
- Fluid Dynamics is the study of fluids in motion. The main equation required for this is Newton's second law for accelerating bodies, i.e.
- http://www.mne.psu.edu/cimbala/Learning/Fluid/Introductory/what_is_fluid_mechanics.htm
5..The Fluid Mosaic Model
The fluid mosaic model describes the plasma membrane structure as a mosaic of phospholipids, cholesterol, proteins, and carbohydrates
Describe the fluid mosaic model of cell membranes
Lacking an affinity for water; unable to absorb, or be wetted by water, "water-fearing."
Having an affinity for water; able to absorb, or be wetted by water, "water-loving."
Having one surface consisting of hydrophilic amino acids and the opposite surface consisting of hydrophobic (or lipophilic) ones.

The Components and functions of the Plasma Membrane
The principal components of a plasma membrane are lipids (phospholipids and cholesterol), proteins, and carbohydrates attached to some of the lipids and some of the proteins.
The fluid mosaic model of the plasma membrane
The fluid mosaic model of the plasma membrane describes the plasma membrane as a fluid combination of phospholipids, cholesterol, and proteins. Carbohydrates attached to lipids (glycolipids) and to proteins (glycoproteins) extend from the outward-facing surface of the membrane.
Phospholipid aggregation
In an aqueous solution, phospholipids tend to arrange themselves with their polar heads facing outward and their hydrophobic tails facing inward.
The structure of a phospholipid molecule
This phospholipid molecule is composed of a hydrophilic head and two hydrophobic tails. The hydrophilic head group consists of a phosphate-containing group attached to a glycerol molecule. The hydrophobic tails, each containing either a saturated or an unsaturated fatty acid, are long hydrocarbon chains.
Structure of integral membrane proteins
Integral membrane proteins may have one or more alpha-helices that span the membrane (examples 1 and 2), or they may have beta-sheets that span the membrane (example 3).https://www.boundless.com/biology/textbooks/boundless-biology-textbook/structure-and-function-of-plasma-membranes-5/components-and-structure-64/fluid-mosaic-model-327-11464/
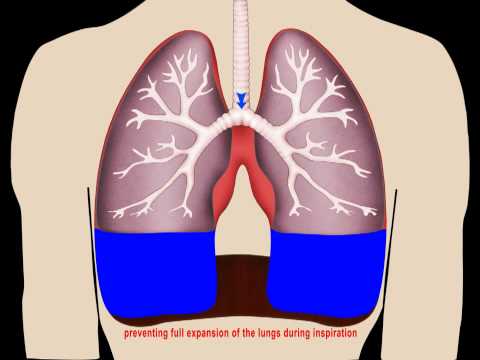
6.Fluid In Lungs
Fluid in the Chest (Pleural Effusion)
What is pleural effusion?
Pleural effusion, also called “water on the lung,” is an excessive buildup of fluid in the space between your lungs and chest cavity. Thin membranes, called pleura, cover the outside of the lungs and the inside of the chest cavity. There’s always a small amount of liquid within this lining to help lubricate the lungs as they expand within the chest during breathing.
Certain medical conditions can cause a pleural effusion.
Pleural effusions are common, with approximately 1.5 million cases diagnosed in the United States every year, according to the American Thoracic Society. It is a serious condition associated with an increased risk of death. One study has shown that 15 percent of hospitalized people diagnosed with pleural effusions die within 30 days.
Certain medical conditions can cause a pleural effusion.
Pleural effusions are common, with approximately 1.5 million cases diagnosed in the United States every year, according to the American Thoracic Society. It is a serious condition associated with an increased risk of death. One study has shown that 15 percent of hospitalized people diagnosed with pleural effusions die within 30 days.
Causes
How does pleural effusion develop?
The pleura creates too much fluid when it’s irritated or infected. This fluid accumulates in the chest cavity outside the lung, causing what’s known as a pleural effusion.
Certain types of cancer can cause pleural effusions. Lung and breast cancer are the most common causes.
Other causes of pleural effusions include:
Certain types of cancer can cause pleural effusions. Lung and breast cancer are the most common causes.
Other causes of pleural effusions include:
- congestive heart failure
- cirrhosis, or poor liver function
- pulmonary embolism, which is caused by a blood clot and is a blockage in the lung arteries
- open heart surgery complications
- pneumonia
- severe kidney disease
Types
Types of pleural effusions
There are several types of pleural effusions, with different causes and treatment options. The first classification of pleural effusions is transudative pleural effusions and exudative pleural effusions.
Complicated pleural effusions, however, contain fluid with significant infection or inflammation. They require prompt treatment that frequently includes chest drainage.
Transudative pleural effusions
This type is caused by fluid leaking into the pleural space as a result of either a low blood protein count or increased pressure in the blood vessels. Its most common cause is congestive heart failure.Exudative effusions
This type is caused by:- blocked lymph or blood vessels
- inflammation
- tumors
- lung injury
Complicated and uncomplicated pleural effusions
There are also complicated and uncomplicated pleural effusions. Uncomplicated pleural effusions contain fluid without signs of infection or inflammation. They’re much less likely to cause permanent lung problems.Complicated pleural effusions, however, contain fluid with significant infection or inflammation. They require prompt treatment that frequently includes chest drainage.
Symptoms
Symptoms and signs of pleural effusion
Some people show no symptoms of pleural effusion. These people usually find out they have the condition through chest X-rays or physical examinations ordered for another reason.
Common symptoms of pleural effusion include:
Common symptoms of pleural effusion include:
- chest pain
- dry cough
- fever
- difficulty breathing when lying down
- shortness of breath
- difficulty taking deep breaths
- persistent hiccups
Diagnosis
Diagnosing pleural effusion
Your doctor will perform a physical examination and listen to your lungs with a stethoscope. He or she may also order a chest X-ray to help diagnose pleural effusion. Other possible tests include:
Your doctor may schedule a thoracoscopy if they discover you have a pleural effusion, but they’re unable to diagnose which type. A thoracoscopy is a surgical procedure that lets the doctor see inside the chest cavity using a fiber optic camera.
Your doctor will make a few small incisions in the chest area while you’re under general anesthesia. Then they’ll insert the camera through one incision and the surgical tool through the other incision to extract a small amount of fluid or tissue for analysis.
- CT scan
- chest ultrasound
- pleural fluid analysis
Your doctor may schedule a thoracoscopy if they discover you have a pleural effusion, but they’re unable to diagnose which type. A thoracoscopy is a surgical procedure that lets the doctor see inside the chest cavity using a fiber optic camera.
Your doctor will make a few small incisions in the chest area while you’re under general anesthesia. Then they’ll insert the camera through one incision and the surgical tool through the other incision to extract a small amount of fluid or tissue for analysis.
Treatment
Treating pleural effusion
The underlying cause of the condition and the severity of the effusion will determine treatment.
Other treatments may be necessary to manage fluid buildup if cancer is the cause of the pleural effusion.
Draining fluid
Generally, treatment involves draining the fluid from the chest cavity, either with a needle or a small tube inserted into the chest. You’ll receive a local anesthetic before this procedure, which will make the treatment more comfortable. You may feel some pain or discomfort at the incision site after the anesthetic wears off. Most doctors will prescribe medication to help relieve pain. You may need this treatment more than once if fluid re-collects.Other treatments may be necessary to manage fluid buildup if cancer is the cause of the pleural effusion.
Pleurodesis
Pleurodesis is a treatment that creates mild inflammation between the lung and chest cavity pleura. After drawing the excess fluid out of the chest cavity, a doctor injects a drug into the area. The drug is often talc. This medication causes the two layers of the pleura to stick together and prevents the buildup of fluid between the two layers by getting rid of the space between them.Surgery
In more serious cases, a doctor surgically inserts a shunt, or small tube, into the chest cavity. This helps redirect the fluid from the chest to the abdomen, where it can be easily removed. Pleurectomy, in which part of the pleural lining is surgically removed, is also an option in very severe cases.
Treatment complications
Risks of pleural effusion treatment
Treatment for minor cases of pleural effusion is minimally invasive. Most people recover within a few days. Minor complications from treatment include slight pain and discomfort, which often go away with time. Some cases of pleural effusion can have more serious complications, depending on the severity of the condition and treatment used.
Serious complications include:
Serious complications include:
- pulmonary edema or fluid in the lungs, which can result from draining fluid too quickly during thoracentesis
- partial collapsed lung
- infection or bleeding
Pleural effusions and cancer
Pleural effusions can be the result of cancer cells spreading to the pleura. They can also be the result of cancer cells blocking the flow of normal fluid within the pleura. Fluid may also build up as a result of certain cancer treatments, such as radiation therapy or chemotherapy.
Certain cancers are more likely to cause pleural effusions than others, including:
In addition to treating the pleural effusion, your doctor will treat the cancer that caused it. Pleural effusions are typically the result of metastatic cancer.
People who are undergoing treatment for cancer may also have compromised immune systems, making them more prone to infections or other complications.
Certain cancers are more likely to cause pleural effusions than others, including:
- lung cancer
- breast cancer
- ovarian cancer
- leukemia
- melanoma
- cervical cancer
- uterine cancer
- shortness of breath
- cough
- chest pain
In addition to treating the pleural effusion, your doctor will treat the cancer that caused it. Pleural effusions are typically the result of metastatic cancer.
People who are undergoing treatment for cancer may also have compromised immune systems, making them more prone to infections or other complications.
Outlook
What is the outlook for a pleural effusion?
Pleural effusions can be serious and life-threatening. Many require hospitalized treatment and some require surgery. The time it takes to recover from pleural effusions depends on the cause, size, and severity of the effusion, as well as your overall health.
You will begin your recovery in the hospital, where you’ll receive the necessary medication and care to help you begin to recover. Many people report feeling tired and weak in the first week after they’ve been discharged from the hospital. On average, you will see your incision sites from surgery heal within two to four weeks.
You will begin your recovery in the hospital, where you’ll receive the necessary medication and care to help you begin to recover. Many people report feeling tired and weak in the first week after they’ve been discharged from the hospital. On average, you will see your incision sites from surgery heal within two to four weeks.





No comments:
Post a Comment